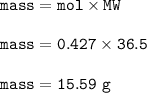Mass of HCl produced :15.59 g
Further explanation
Reaction
2NaCl + H₂SO₄ → 2HCl + Na₂SO₄
Excess = H₂SO₄
Limiting = NaCl
mass NaCl = 25 g
mol NaCl(MW=58.5 g/mol) :
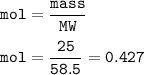
From the equation, mol ratio NaCl : HCl = 2 : 2, so mol HCl = mol NaCl =0.427
Mass of HCl(MW=36.5 g/mol) :