Answer:
1 mole
Step-by-step explanation:
Given parameters:
Mass of sulfur = 32.066g
Unknown:
Number of moles of sulfur = ?
Solution:
The number of moles of sulfur can be determined using;
Number of moles =
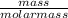
Molar mass of sulfur = 32g/mol
Number of moles =
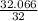 = 1 mole
= 1 mole