Answer:
Step-by-step explanation:
Given that,
The mass of a sample, m = 16.7 grams
Volume of the sample, V = 4.4 cm³
We need to find the density of the metal sample.
Density = mass/volume
Substituting all the values,

So, the density of the metal sample is
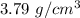 .
.