Answers:
1.) Lithium and Sulfide:
- Formula:
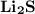
- Ion Charges:
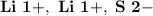
2.) Lithium and Chlorine:
- Formula:
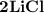
- Ion Charges:
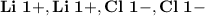
3.) Lithium and Oxygen:
- Formula:
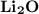
- Ion Charges:
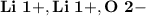
4.) Lithium and Nitrogen:
- Formula:
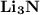
- Ion Charges:
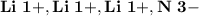
5.) Magnesium and Sulfur:
- Formula:
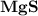
- Ion Charges:
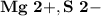
6.) Magnesium and Chlorine:
- Formula:
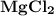
- Ion Charges:
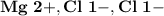
7.) Magnesium and Oxygen:
- Formula:
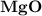
- Ion Charges:
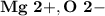
8.) Magnesium and Nitrogen:
- Formula:
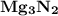
- Ion Charges:
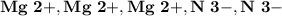
Step-by-step explanation:
______________________________
Lithium and Sulfur:
- In order to make Lithium Sulfide, There must be 2 Lithium and 1 Sulfur. You transfer the electrons from both Lithium's to the Sulfur.
Lithium and Chlorine:
- In order to make Lithium Chloride, There must be 2 Lithium and 2 Chlorine. You transfer the electrons from both Lithium's to the Chlorines, (One electron for each chlorine.)
Lithium and Oxygen:
- In order to make Lithium Oxide, There must be 2 Lithium and 1 Oxygen. You transfer the electrons from both Lithium to Oxygen.
Lithium and Nitrogen:
- In order to make Lithium Nitride, There must be 3 Lithium and 1 Nitrogen. You transfer the electrons from all 3 Lithium to Nitrogen.
Magnesium and Sulfur:
- In order to make Magnesium Sulfide, There must be 1 Magnesium and 1 Sulfur. You transfer the both electrons from Magnesium to Sulfur.
Magnesium and Chlorine:
- In order to make Magnesium Chloride, There must be 1 Magnesium and 2 Chlorine. You transfer on electron to each Chlorine.
Magnesium and Oxygen:
- In order to make Magnesium Oxide, There must be 1 Magnesium and 1 Oxygen. You transfer both electrons from Magnesium to Oxygen.
Magnesium and Nitrogen:
- In order to make Magnesium Nitride, There must be 3 Magnesium and 2 Nitrogen. You transfer 3 electrons from Magnesium to Nitrogen.
______________________________