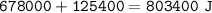Energy lost to condense = 803.4 kJ
Further explanation
Condensation of steam through 2 stages:
1. phase change(steam to water)
2. cool down(100 to 0 C)
1. phase change(condensation)
Lv==latent heat of vaporization for water=2260 J/g
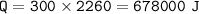
2. cool down
c=specific heat for water=4.18 J/g C
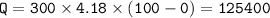
Total heat =