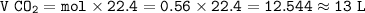Volume of CO₂ obtained : 13 L
Further explanation
Reaction
C₂H₅OH + 3 O₂ ⇒ 2 CO₂ + 3 H₂0 + 8842 Joules
moles of ethanol=0.28
From equation, mol ratio ethanol : CO₂ = 1 : 2, so mol CO₂ :
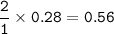
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters / mol.
Then volume of CO₂ :