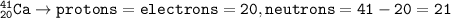Ca-41 has 20 electrons
Further explanation
The elements in nature have several types of isotopes
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Calcium is an alkaline earth metal element that is in group 2 and has the atomic number 20
The isotope of Ca-41 :
Number of protons=number of electrons=number of atoms(neutral atom)