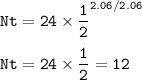Number of atoms of Cesium-134 left = 12 atoms
Further explanation
The atomic nucleus can experience decay into 2 particles or more due to the instability of its atomic nucleus.
Usually radioactive elements have an unstable atomic nucleus.
The main particles are emitted by radioactive elements so that they generally decay are alpha (α), beta (β) and gamma (γ) particles
General formulas used in decay:
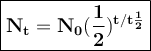
t = duration of decay
t 1/2 = half-life
N₀ = the number of initial radioactive atoms
Nt = the number of radioactive atoms left after decaying during T time
No=24 atoms
t1/2=2.06 years
T=2.06 years = t1/2
Since the decay time is the same as the half-life time, the number of atoms of Cesium-134 will be halved from 24 to 12.
Or we can use the formula above