Volume of NaOH required to react = 145.5 ml
Further explanation
Reaction
CO₂(g) + 2 NaOH(aq) ⇒Na₂CO₃(aq) + H₂O(l)
The volume of CO₂ : 0.45 L
mol CO₂ at STP (O C, 1 atm) ⇒ at STP 1 mol gas 22.4 L :
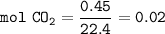
From the equation, the mol ratio of CO₂ : NaOH = 1 : 2, so mol NaOH :
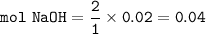
Then volume of NaOH :
