Answer:
4Al + 3O₂ → 2Al₂O₃
Step-by-step explanation:
The problem here is in balancing the equation below:
Al + O₂ → Al₂O₃
In balancing a chemical equation, the number of atoms on both sides of the expression must be the same;
put the alphabets a, b and c before each specie;
aAl + bO₂ → cAl₂O₃
Conserving Al;
a = 2c
Conserving O:
2b = 3c
Now, let c = 1, a = 2
b =
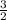
Multiply through by 2;
c = 2, a = 4 and b = 3
4Al + 3O₂ → 2Al₂O₃