Answer:
2N₂ + O₂ → 2N₂O
Step-by-step explanation:
Given equation:
N₂ + O₂ → N₂O
In balancing a chemical equation, the number of atoms on both sides of the expression must be the same.
To solve this problem, we use a simple mathematical approach;
put coefficients a,b and c
aN₂ + bO₂ → cN₂O
Conserving N;
2a = 2c
Conserving O:
2b = c
Let a = 1, c = 1 , b =
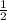
multiply through by 4
a = 2, b = 1 and c = 2
Balanced equation is;
2N₂ + O₂ → 2N₂O