The 32.06 represent The atomic mass, the average number of protons & neutrons
Further explanation
The Atomic Number (Z) indicates the number of protons in an atom of an element. If the atom is neutral then the number of protons will be equal to the number of electrons. So the atomic number can also indicate the number of electrons.
So atomic number = number of protons = number of electrons
Mass Number (A) is the sum of protons and neutrons
Mass Number (A) = Number of protons + Number of Neutrons
So that the relationship between atomic numbers and mass numbers can be formulated as follows:
Atomic Number (Z) = Mass Number (A) - Number of Neutrons
In the following element notation,
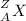
X = symbol of elemental atom
A = mass number
= number of protons + number of neutrons
Z = atomic number
= number of protons = number of electrons, on neutral elements