Answer:
When the pressure and the temperature are increased the volume is 285.7 ml.
Step-by-step explanation:
We can find the new volume by using the Ideal Gas Law:
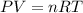
Where:
P: is the pressure
V: is the volume
n: is the number of moles
R: is the gas constant
T: is the temperature
Initially, when V₁ = 200 ml, P₁ = 500 torr and T₁ = 10 °C, we have:
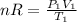 (1)
(1)
And finally, when P₂ = 700 torr and T₂ = 20 °C, we have:
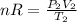 (2)
(2)
By equating (1) with (2):
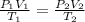
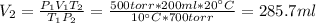
Therefore, when the pressure and the temperature are increased the volume is 285.7 ml.
I hope it helps you!