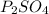Answer:
See the answer below
Step-by-step explanation:
P can displace Q from its salt solution means that P is more reactive than Q.
Q cannot displace R from its salt solutions means that R is more reactive than Q.
a) There is no information as to which of P and R has the capacity to displace one another from their salt solutions. Hence, the most reactive metal can be either of P and R.
b) When P displaces Cu from CuSO4 solution, the equation can be represented below (assuming that P is monoatomic):

Hence, the product formed is