Answer:
The volume of the ideal gas on another planet will be 6.7 m³.
Step-by-step explanation:
We can find the volume occupied by the ideal gas on another planet by using the Ideal Gas Law:
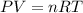
Where:
P: is the pressure
V: is the volume
n: is the number of moles
R: is the gas constant = 8.206x10⁻⁵ m³ atm K⁻¹mol⁻¹
T: is the temperature
Since the gas occupies a volume of 8.7 m³ with a pressure of 6 atm and temperature 4.8 °C on earth, we have the following number of moles:
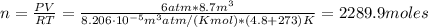
Now we can calculate the volume occupied by the ideal gas on another planet:
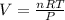
With T = 8.7 °C and P = 7.9 atm
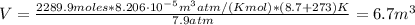
Therefore, the volume of the ideal gas on another planet will be 6.7 m³.
I hope it helps you!