Answer:
ΔQ = - 762 J (Negative Sign shows heat transfer from the system)
Step-by-step explanation:
The heat transferred to or from the system can be given by using First law of Thermodynamics as follows:
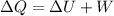
where,
ΔQ = Heat Transferred to or from system = ?
ΔU = Change in Internal Energy of System = Change in Thermal Energy
ΔU = - 240 J (negative sign due to decrease in energy)
W = Work done on system = -522 J (negative sign due to work done "on" system)
Therefore,
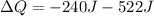
ΔQ = - 762 J (Negative Sign shows heat transfer from the system)