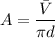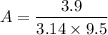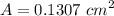Solution :
Assume that the diameter of the watch glass is 9.5 cm.
There are 10 drops of hexagon in 1 mL.
V = volume of hexagon in per drop =
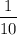 mL per drop
mL per drop
V = 0.1 mL per drop
N = number of drops to form phospholipid layer
N = 39 (assumed)
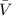 = volume of hexagon required to form phospholipid layer
= volume of hexagon required to form phospholipid layer
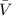 = V x N
= V x N
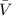 = 0.1 x 39
= 0.1 x 39
= 3.9 mL
Since 1 mL = 1
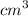
∴
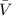 = 3.9
= 3.9
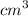
d = diameter of the watch glass = 9.5 cm
A = area of one molecule