Answer:
The maximum speed of the ejected photoelectrons is 1.815 x 10⁶ m/s.
Step-by-step explanation:
Given;
frequency of the light, f = 3.5 x 10¹⁵ Hz
work function of the metal, Φ = 5.11 eV
Φ = 5.11 x 1.602 x 10⁻¹⁹ J = 8.186 x 10⁻¹⁹ J
The energy of the incident light is given as sum of maximum kinetic energy and work function of the metal.
E = K.E + Φ
where;
E is the energy of the incident light, calculated as;
E = hf
E = (6.626 x 10⁻³⁴)(3.5 x 10¹⁵)
E = 2.319 x 10⁻¹⁸ J
The maximum kinetic energy of the photoelectrons is calculated as;
K.E = E - Φ
K.E = 2.319 x 10⁻¹⁸ J - 8.186 x 10⁻¹⁹ J
K.E = 2.319 x 10⁻¹⁸ J - 0.8186 x 10⁻¹⁸ J
K.E = 1.5004 x 10⁻¹⁸J
The maximum speed of the ejected photoelectrons in 10⁶ m/s is given as;
K.E = ¹/₂mv²
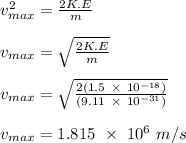
Therefore, the maximum speed of the ejected photon-electrons is 1.815 x 10⁶ m/s.