Answer:
the initial concentration of SCN- in the mixture is 0.00588 M
Step-by-step explanation:
The computation of the initial concentration of the SCN^- in the mixture is as follows:
As we know that
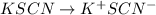
As it is mentioned in the question that KSCN is present 10 mL of 0.05 M
So, the total milimoles of SCN^- is
= 10 × 0.05
= 0.5 m moles
The total volume in mixture is
= 45 + 10 + 30
= 85 mL
Now the initial concentration of the SCN^- is
= 0.5 ÷ 85
= 0.00588 M
hence, the initial concentration of SCN- in the mixture is 0.00588 M