Answer:
There are 1.8 moles of aluminum.
Step-by-step explanation:
To solve this problem we need to convert moles of pyrophillite (Al₂Si₄O₁₀(OH)) into moles of aluminum (Al):
- In one mol of Al₂Si₄O₁₀(OH) there are two moles of Al.
With the above information in mind we can now do the conversion:
- 0.900 moles Al₂Si₄O₁₀(OH) *
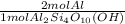 = 1.8 mol Al
= 1.8 mol Al
Thus the answer is that there are 1.8 moles of aluminum.