Answer: The molar mass of gas is 48.4 g/mol
Step-by-step explanation:
To calculate the relation of density and molar mass of a compound, we use the ideal gas equation:
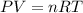
P = pressure = 851.2 mm Hg = 1.12 atm ( 760 mm Hg = 1atm)
V = Volume
n = number of moles
R = gas constant = 0.0821 Latm/Kmol
T = temperature =
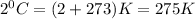
Number of moles (n) can be written as:
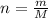
where, m = given mass
M = molar mass
where,
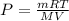
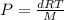
where d = density
The relation becomes:
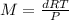
Putting the values we get :
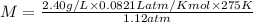
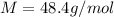
Thus molar mass of gas is 48.4 g/mol