Answer:
The total heat required to completely turn 20 g of ice at -20°C to steam, is 65880 J
Explanation:
The given parameters are;
The mass of the ice = 20 g
The initial temperature of the ice = -20°C
The specific heat capacity of the ice, ΔH(fusion) = 2.03 J/(g·°C)
The latent heat of fusion of ice = 334 J/g
The specific heat capacity of the water = 4.184 J/(g·°C)
The latent heat of evaporation of water = 2501 J/g
The heat required to raise the temperature of the ice to from -20°C to 0°C, Q₁, is given as follows;
Q₁ = 20 × 2.03 × (0 - (-20)) = 812
Q₁ = 812 J
The latent heat required to melt the 20 g of ice at 0°C is given as follows;
Q₂ = ΔH(fusion) × mass, m = 334 × 20 = 6680
Q₂ = 6680 J
The heat, Q₃, required to raise the temperature of the ice to 100°C (boiling point temperature) is given as follows;
Q₃ = 20 × 4.184 × (100 - 0) = 8368
Q₃ = 8368 J
The heat, Q₄, required to evaporate the 20 g of water at 100°C is given as follows
Q₄ = 2501 × 20 = 50020
Q₄ = 50020 J
The total heat,
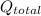 required to completely turn 20 g of ice at -20°C to steam, is given as follows;
required to completely turn 20 g of ice at -20°C to steam, is given as follows;
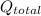 = Q₁ + Q₂ + Q₃ + Q₄ = 812 + 6680 + 8368 + 50020 = 65880
= Q₁ + Q₂ + Q₃ + Q₄ = 812 + 6680 + 8368 + 50020 = 65880
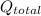 = 65880 J.
= 65880 J.