Answer:
Decrease the number of gas particles, increase the object's area, and reduce the temperature of the gas
Step-by-step explanation:
TEMPERATURE:
Decreasing the temperature will slow down the molecules. Hence, less no. of collisions will take place between walls of object and molecules. This will result in decrease of pressure.
Therefore, the pressure of a gas can be decreased by increasing its temperature.
NUMBER OF GAS PARTICLES:
Decreasing the number of particles will result in less no. of collisions, hence decreasing the pressure.
Therefore, the pressure of a gas can be decreased by decreasing its no. of molecules or no. of particles.
AREA OF OBJECT:
The pressure is given by the formula:
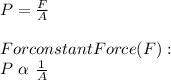
where,
A = Area of Object
Therefore, the pressure of a gas can be decreased by increasing area of object.
So, the correct option is:
Decrease the number of gas particles, increase the object's area, and reduce the temperature of the gas