We know that:
One mole of any element or compound contains 6.022*10²³ atoms/molecules
where 6.022*10²³ is also known as Avogadro's number
So, Number of moles =
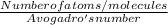
Number of moles in the given sample of H₂O:
From above:
Number of moles = Number of molecules / Avogadro's number
replacing the variables
Number of moles =
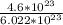
Number of moles =
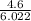
Number of moles = 0.7639 moles
Number of Moles in correct significant Figures:
We know that the number of significant figures of the quotient is the least number of significant figures from the numbers being divided
To find the number of moles, we divided 4.6 by 6.022
Here, 4.6 has 2 significant figures and 6.022 has 4 significant figures
Hence, the quotient(number of moles) will have 2 significant figures
Number of moles in correct significant figures = 0.76 moles