Answer:
Step-by-step explanation:
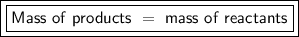
The Law of Conservation of Mass states that mass cannot be created or destroyed.
In a chemical reaction, there are products and reactants. The reactants react and produce the products.
Since mass can't be created or destroyed, the mass of the products must be equal to the mass of the reactants if mass was conserved in a chemical reactions.