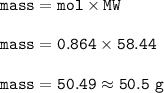The mass of NaCl : 50.5 g
Further explanation
The mole is the number of particles(molecules, atoms, ions) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
There are 5.20 x 10²³ units of NaCl, so mol NaCl :
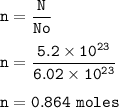
Mass of NaCl(MW=71 g/mol) :