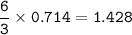FeBr₃ ⇒ limiting reactant
mol NaBr = 1.428
Further explanation
Reaction
2FeBr₃ + 3Na₂S → Fe₂S₃ + 6NaBr
Limiting reactant⇒ smaller ratio (mol divide by coefficient reaction)
211 g of Iron (III) bromide(MW=295,56 g/mol), so mol FeBr₃ :
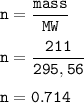
186 g of Sodium sulfide(MW=78,0452 g/mol), so mol Na₂S :
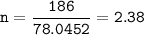
Coefficient ratio from the equation FeBr₃ : Na₂S = 2 : 3, so mol ratio :
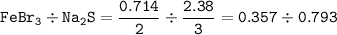
So FeBr₃ as a limiting reactant(smaller ratio)
mol NaBr based on limiting reactant (FeBr₃) :