Answer:
1.45 x 10²²atoms
Step-by-step explanation:
A formula unit is the number of elementary particles a specie contains.
A mole of any substance contains 6.02 x 10²³ number of particles. The particles can be atoms, molecules, formula units, electrons, protons and so forth.
To solve this problem, we find the number of moles from the given mass first.
Number of moles =

Molar mass of Cu(NO₃)₂ = 63.5 + 2(14 + 3(16)) = 187.5g/mol
Number of moles =
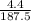 = 0.024mole
= 0.024mole
Now,
Number of formula units = 0.024 x 6.02 x 10²³ = 1.45 x 10²²atoms