Answer:
0.879 mg
Step-by-step explanation:
To solve this problem we need to use the definition of ppm:
- ppm = μg solute / g solution
With the above information in mind we can calculate the micrograms of lead using the concentration (13.97 ppm) and mass of solution (62.9 g):
- μg lead = 13.97 ppm * 62.9 g
Finally we convert μg to mg:
- 878.713 μg *
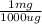 = 0.879 mg of lead
= 0.879 mg of lead