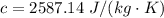Complete Question
An unknown material, m1 = 0.41 kg, at a temperature of T1 = 86 degrees C is added to a Dewer (an insulated container) which contains m2 = 1.7 kg of water at T2 = 22 degrees C. Water has a specific heat of cw = 4186 J/(kg⋅K). After the system comes to equilibrium the final temperature is T = 30.3 degrees C.
Part (a) Input an expression for the specific heat of the unknown material.
Part (b) What is the specific heat in J/(kg⋅K)?
Answer:
a
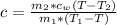
b
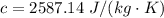
Step-by-step explanation:
From the question we are told that
The mass of the material is
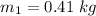
The temperature is
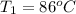
The mass of water is
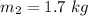
The temperature of water is
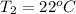
The specific heat of water is
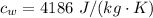
The temperature of the system is
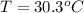
Generally heat lost by the unknown material = heat gained by water
Generally the heat gained by water is mathematically represented as
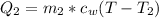
=>
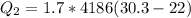
=>
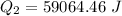
Generally the heat lost by the unknown material is mathematically represented as
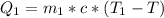
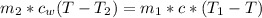
=>
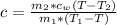
Here c is the specific heat capacity of the unknown material
=>
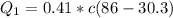
=>
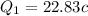
So
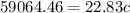
=>
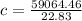
=>