Answer:
6282 kJ
Step-by-step explanation:
Given that:
The mass (m) = 15 kg
The specific latent heat of ice fusion
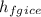 = 335 kJ/kg
= 335 kJ/kg
The specific heat capacity of water = 4.19 kJ/kg.c
The initial temperature of the ice
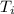 = 0° C
= 0° C
The final temperature of the water
 = 20° C
= 20° C
To find the needed amount of heat to convert 0° C ice to 20° C of water.
To do that, we need to find the latent heat required for the phase change from 0° C ice to 0° C water, then the heat required to convert 0° C water to 20° C water.
Heat required =

Heat required = (15 × 335) + (15 × 4.19) × (20 - 0)
Heat required = 5025 + 62.85 × 20
Heat required = 5025 + 1257
Heat required = 6282 kJ