Answer:
The theoretical yield of carbonic acid is 1.24 grams.
Step-by-step explanation:
The balanced equation for the formation of carbonic acid by the reaction of carbon dioxide with water is:
CO₂ + H₂O → H₂CO₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of reactant and product participate:
- CO₂: 1 mole
- H₂O: 1 mole
- H₂CO₃: 1 mole
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P * V = n * R * T
Moles of carbon dioxide can be calculated using ideal gas law equation. In this case:
- P= 101.3 kPa= 1 atm
- V= 495 mL= 0.495 L (being 1,000 mL=1 L)
- n=?
- R= 0.082
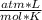
- T= 25 C= 298 K (being 0 C=273 K)
Replacing:
1 atm* 0.495 L= n* 0.082
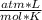 *298 K
*298 K
Solving:
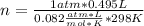
n= 0.02 moles
Then, by reaction stoichiometry 0.02 moles of carbon dioxide produces 0.02 moles of carbonic acid.
Since the molar mass of carbonic acid is 62.03 g/mol, then you can apply the following rule of three: if there are 62.03 grams in 1 mole, how much mass is there in 0.02 moles?
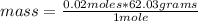
mass= 1.24 grams
The theoretical yield of carbonic acid is 1.24 grams.