Answer:
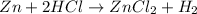
Step-by-step explanation:
A chemical reaction is said to have occured when reactants react to form new products where the atoms undergo a change in bonding.
From the given reactions:
1.
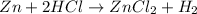
Here Zn combines with hydrochloric acid to form zinc chloride and hydrogen.
2.
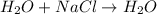
Here water remains as such as no new substance is formed.
3.
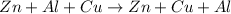
Here no new substance is formed.
4.
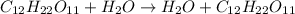
Here no new substance is formed.
Thus
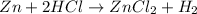 demonstrates an actual chemical reaction that forms a new substance
demonstrates an actual chemical reaction that forms a new substance