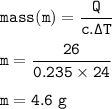The mass of the silver : 4.6 g
Further explanation
Heat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C
∆T = temperature difference, ° C / K
∆T = temperature difference :
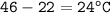
The specific heat of silver is 0.235 J/gºC
Q = heat = 26 J
The mass :