Hey There!
_____________________________________
Answer:
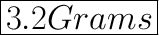
_____________________________________
Moles:
Moles is the unit which measures the amount of substance in the System International (SI). A mole is the amount of substance that contains the same amount of substance as the amount of substance exactly in exactly 12 g of carbon-12. C-12 is the standard to measure moles.
I have attached the Equations for moles.
_____________________________________
For this question we will use the formula,
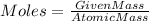
Rearrange the equation,
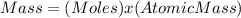
Given:
Moles = 0.2
Molecular Mass of Oxygen Atom(Not molecule) = 16
Thus,
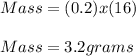
The mass of 0.2 moles of oxygen atom is 3.2 grams.
_____________________________________
Best Regards,
'Borz'