Given that,
Mass of water, m = 5 kg
The temperature increases from 20℃ to 40℃.
Specific heat capacity of water is 4200 J kg⁻¹ k⁻¹
To find,
Heat energy required to raise the temperature.
Solution,
The formula for the heat energy required to raise the temperature is given by :
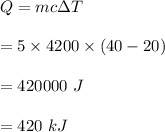
So, the heat energy required is 420 kJ.