Answer:
m = 35 g = 0.035 kg
Step-by-step explanation:
The amount of heat required by a specific mass of wax to be melted at its melting point can be given by the following formula:
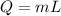
where,
Q = Heat Supplied by the Heater = 7700 J
m = mass of wax melted = ?
L = Latent Heat of Fusion of Wax = 220 J/g
Therefore,
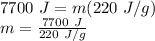
m = 35 g = 0.035 kg