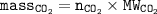To determine the mass of CO₂, the following must be known :
- the molar mass of CaCO₃
- the mole ratio of CaCO₃ to CO₂
- the molar mass of CO₂
Further explanation
Reaction
Decomposition of CaCO₃
CaCO₃ ⇒ CaO + CO₂
Given the mass of CaCO₃, so to determine the mass of CO₂ :
1. Find the mol of CaCO₃ from the molar mass of CaCO₃
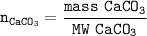
2. Find the mole ratio of CaCO₃ : CO₂(from equation = 1 : 1)
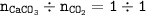
3. Find the mass of CO₂ from the molar mass of CO₂