Answer:
Density = 0.17 g/L
Step-by-step explanation:
It is given that,
Volume of the inflated balloon filled with Helium,
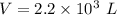
Mass, m = 374 g
We need to find the density of helium. It is equal to its mass per unit volume. It can be given by :
d =m/V
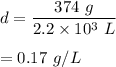
So, the density of helium in the balloon is 0.17 g/L.