Answer:
yes the Bohr model predicts their spectra accurately
Step-by-step explanation:
The Bohr model based on one electron system
The atom H has a single atom and the ion Be+3 has a single atom as well and this proves that the Bohr model predicts their spectra accurately .
also apply the equation model to further explain
E =
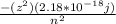
z = atomic number
for H the value of z = 1
for Be+3 the value of z = 4
when this values are substituted into the equation above
E =
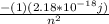 , E =
, E =
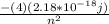
this results show that the energy level of Be+3 is higher than the energy level of H by a factor of 16 , and this shows that their line patterns are similar