The number of atoms N = 5.8 x 10²¹
Further explanation
A mole is a unit of many particles (atoms, molecules, ions) where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12
1 mole = 6.02.10²³ particles
mass of N in 0.82 g of NaNO₃ (MW NaNO₃: 85 g/mol) :

moles of N :
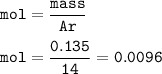
The number of atoms N :
