Answer:
V₂ = 23.18 L
Step-by-step explanation:
Given that,
Initial volume of a balloon, V₁ = 8.5 L
Initial pressure, P₁ = 150 kPa
We need to find new volume when the pressure drops to 55 kPa.
It is based on the concept of Boyle's law. The mathematical form of the Boyle's law is given by :
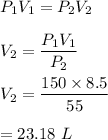
So, the new volume is 23.18 L.