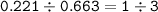The empirical formula of metal iodide : CoI₃(Cobalt(III) Iodide)
Further explanation
13.02 g sample of Cobalt , then mol Co(MW=58.933 g/mol) :
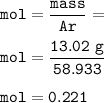
Mass of metal iodide formed : 97.12 g, so mass of Iodine :

Then mol iodine (MW=126.9045 g/mol) :
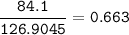
mol ratio of Cobalt and Iodine in the compound :