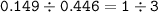The empirical formula : FeBr₃
Further explanation
A 8.310 g sample of Iron, so mol of Iron(Ar=55.845 g/mol) :
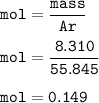
Mass of metal bromide formed : 43.98 g, so mass of Bromine :

then mol Bromine (Ar=79,904 g/mol) :
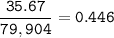
mol ratio of Iron and Bromine in the compound :