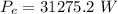Answer:
The value is
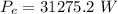
Step-by-step explanation:
From the question we are told that
The efficiency of the carnot engine is
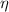
The efficiency of a heat engine is
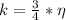
The operating temperatures of the carnot engine is
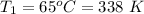 to
to
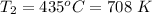
The rate at which the heat engine absorbs energy is
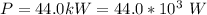
Generally the efficiency of the carnot engine is mathematically represented as
![\eta = [ 1 - (T_1 )/(T_2) ]](https://img.qammunity.org/2021/formulas/physics/college/ldicb63t556arqo50xe9adzg9bb82vw99q.png)
=>
![\eta = [ (T_2 - T_1)/(T_2) ]](https://img.qammunity.org/2021/formulas/physics/college/bmrkxdxdx9cuwlagefmr3yt49bbnaa3ta4.png)
=>
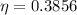
Generally the efficiency of the heat engine is
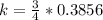
=>
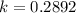
Generally the efficiency of the heat engine is also mathematically represented as
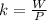
Here W is the work done which is mathematically represented as
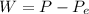
Here
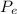 is the heat exhausted
is the heat exhausted
So
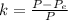
=>
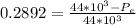
=>