Answer:
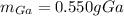
Step-by-step explanation:
Hello!
In this case, since the applied current for the 50.0 mins provides the following charge to the system:
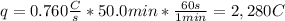
As 1 mole of electrons carries a charge of 1 faraday, or 96,485 coulombs, we can compute the moles of electrons involved during the reduction:
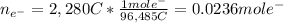
Then the reduction of Ga³⁺ to Ga involves the transference of three electrons, we are able to compute the moles and therefore the mass of deposited gallium:
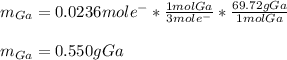
Best regards!