Answer:
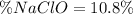
Step-by-step explanation:
Hello!
In this case, since the percent of any substance into a mixture containing it, is computed by dividing the mass of the substance and the mass of the whole mixture, for 3.86 g of pure sodium hypochloride (bleach's active component) dissolved in 35.9 g of bleach, the percent turns out:
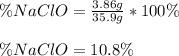
Best regards!