Answer:
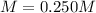
Step-by-step explanation:
Hello!
In this case, since the molarity of a solution is computed by dividing the moles of solute by the volume of solution in liters, we first need to compute the moles of solute knowing that the molar mass of calcium hydroxide is 74.1 g/mol as follows:
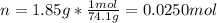
Next, since the 100-mL solution is also expressed in liters by 0.100 L, we directly compute the molarity as shown below:
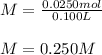
Which is expressed in molar units that are mol/L.
Best regards!