Answer:
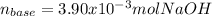
Step-by-step explanation:
Hello!
In this case, since the sulfuric acid and sodium hydroxide react in a 1:2 mole ratio, given the reaction, we realize they have the following mole ratio at the equivalence point:
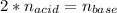
Which in terms of concentrations and volumes is:
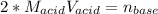
Thus, we can plug in the volume and concentration of acid to find the moles of base:

Best regards!