Answer:
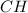
Step-by-step explanation:
Hello!
In this case, given the percent composition of C and H in acetylene, in order to find its empirical formula we compute moles of C and H assuming those percentages as masses:
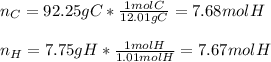
Next, since they have the same number of moles we infer they are in a 1:1 subscript ratio on the empirical formula, where is therefore:
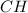
Best regards!